Research
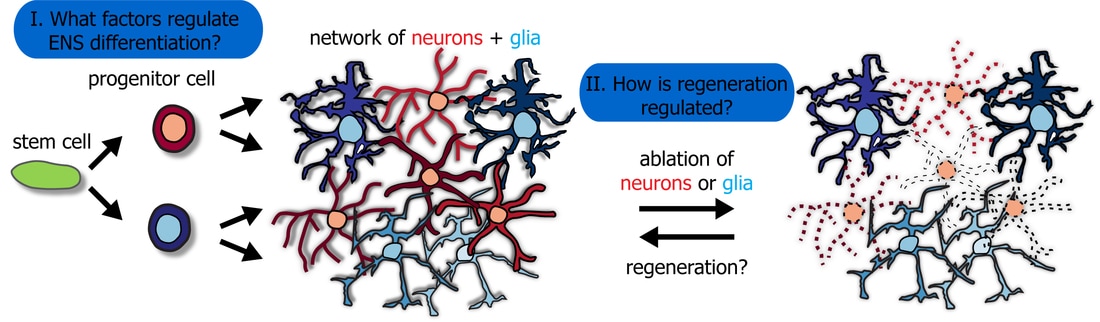
Our long-term goal is to understand how the formation of the nervous system from stem cells to a complex network of neurons and glia is regulated during normal development in situations that model human diseases, and under regenerating conditions. Our work focuses on the enteric nervous system (ENS), the largest parts of the peripheral nervous system.
|
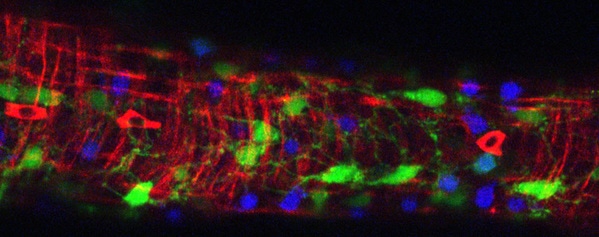
The zebrafish is an excellent vertebrate model system to study ENS development and function due to its rapid development, genetic and embryological tractability, and optical transparency, which makes it highly amenable to in vivo imaging techniques.
Very little is known about how the differentiation of enteric progenitor cells into neuronal and glial cell fates is regulated in the ENS. |
Research in our lab thus strives to answer the following main research questions:
1. Which genes and signaling pathways regulate enteric nervous system development?
2. How is ENS regeneration regulated?
We hope to uncover not only cellular, genetic, and molecular mechanisms underlying cell fate determination, but also contribute to developing therapeutic approaches using stem cells to repair enteric nervous system diseases.
1. Which genes and signaling pathways regulate enteric nervous system development?
2. How is ENS regeneration regulated?
We hope to uncover not only cellular, genetic, and molecular mechanisms underlying cell fate determination, but also contribute to developing therapeutic approaches using stem cells to repair enteric nervous system diseases.
Some of our current projects include:
Regulate enteric nervous system development:
Using cell type specific transcriptional profiling with RNA-seq, we work to find genes and signaling pathways that are active in different ENS cell populations and during different developmental time points to regulate ENS development. To test gene functions, we generate targeted mutations using the CRISPR/Cas9 genome editing technology to unravel the role of these genes and pathways for ENS development. We also use a set of zebrafish mutants with major defects in ENS development to identify novel genes playing a role during ENS development.
Regulation of ENS regeneration:
Zebrafish have an unparalleled ability to regenerate body parts including the brain and thus are widely used as a model to study regeneration. We investigate whether the zebrafish ENS can regenerate after experimental ablation of enteric neurons. And if so, how is this regeneration process regulated?
Regulate enteric nervous system development:
Using cell type specific transcriptional profiling with RNA-seq, we work to find genes and signaling pathways that are active in different ENS cell populations and during different developmental time points to regulate ENS development. To test gene functions, we generate targeted mutations using the CRISPR/Cas9 genome editing technology to unravel the role of these genes and pathways for ENS development. We also use a set of zebrafish mutants with major defects in ENS development to identify novel genes playing a role during ENS development.
Regulation of ENS regeneration:
Zebrafish have an unparalleled ability to regenerate body parts including the brain and thus are widely used as a model to study regeneration. We investigate whether the zebrafish ENS can regenerate after experimental ablation of enteric neurons. And if so, how is this regeneration process regulated?